Exploring the Factors Affecting the Time it Takes for Water to Reach Room Temperature
When you pour a glass of cold water, have you ever wondered how long it takes for it to reach that perfect room temperature? The time it takes for water to transition from its initial temperature to room temperature is influenced by a variety of factors. In this article, we will delve into the science behind this process and examine the key variables that impact the time it takes for water to achieve equilibrium with its surroundings.
I. Understanding Temperature and Heat Transfer
Before we dive into the factors affecting the rate at which water reaches room temperature, let's briefly explore the concepts of temperature and heat transfer.
Temperature is a measure of the average kinetic energy of molecules in a substance.
Heat transfer, on the other hand, is the process by which thermal energy is exchanged between objects of different temperatures.
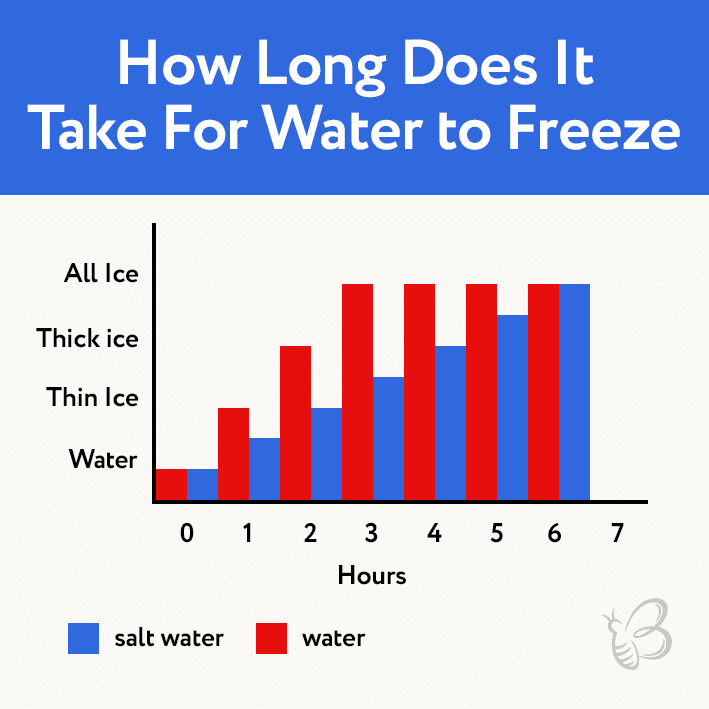
how long does it take for water to get to room temperature
II. Factors Affecting the Rate of Cooling
1. Initial Temperature:
The starting temperature of the water plays a significant role in determining how long it takes for it to reach room temperature.
Water at a higher initial temperature will generally cool down faster than water at a slightly lower temperature.
2. Surface Area and Volume:
The surface area-to-volume ratio of the container holding the water affects the rate of heat transfer.
A container with a larger surface area relative to its volume will facilitate faster heat exchange with the surrounding air, resulting in quicker temperature equalization.
3. Ambient Temperature:
The temperature of the surrounding environment also influences the rate of cooling. Water will reach room temperature faster in a warmer room compared to a colder one.

how long does it take for water to get to room temperature
4. Insulation:
The type and quality of the container insulation can impact the rate of heat loss.
Well-insulated containers will retain heat and slow down the cooling process, while poorly insulated containers will allow heat to escape more rapidly.
5. Convection and Conduction:
Heat can be transferred through convection (movement of heated fluid) and conduction (direct contact between materials). Stirring the water promotes convection, accelerating the heat transfer process.
6. Humidity:
Humidity levels in the environment affect the rate of evaporation, which is a cooling process. Higher humidity can slow down evaporation and consequently delay the cooling of the water.
7. Altitude:
Atmospheric pressure decreases with higher altitude, affecting the boiling point of water. At lower pressures, water boils at lower temperatures, which can impact the rate of cooling.
8. Dissolved Solids:
The presence of dissolved solids, such as salt or sugar, can alter the freezing and boiling points of water, influencing its cooling rate.
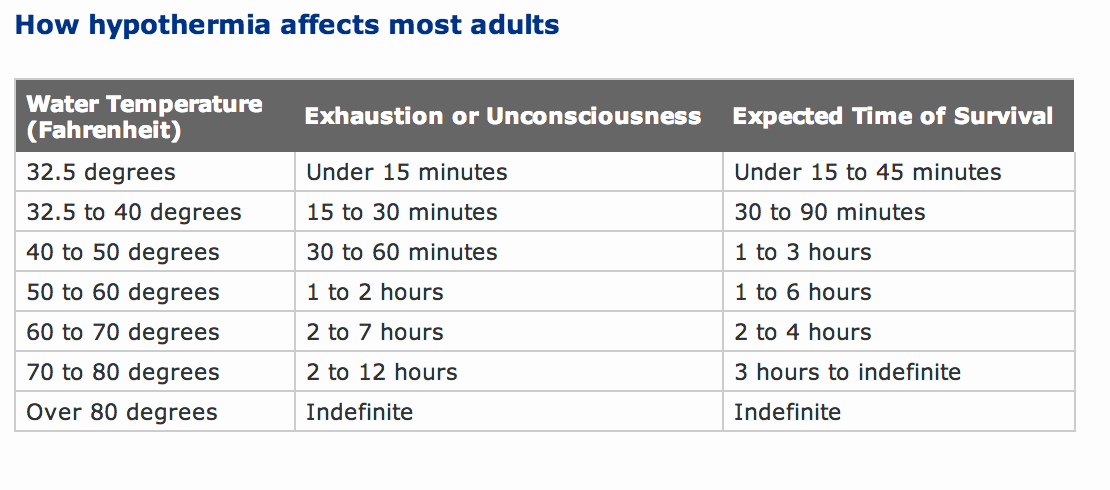
how long does it take for water to get to room temperature
III. Conclusion
In conclusion, the time it takes for water to reach room temperature is influenced by a multitude of factors.
From the initial temperature and container properties to ambient conditions and heat transfer mechanisms, each variable plays a crucial role in determining the rate of cooling.
Whether you're waiting for a refreshing glass of water to warm up or conducting scientific experiments, understanding these factors can provide valuable insights into the fascinating world of heat transfer and thermodynamics.
So, the next time you find yourself pondering over the perfect room temperature water, you'll have a deeper appreciation for the complex interplay of science and nature.