How Many Neutrons Does Neon Have: Understanding Atomic Structure
Neon, a noble gas element with the atomic symbol Ne, is known for its vibrant lights and presence in neon signs. If you're curious about the atomic structure of neon and how many neutrons it contains, this article provides a comprehensive explanation. By exploring the concept of atomic structure and the composition of neon's nucleus, we can gain a better understanding of this fascinating element.
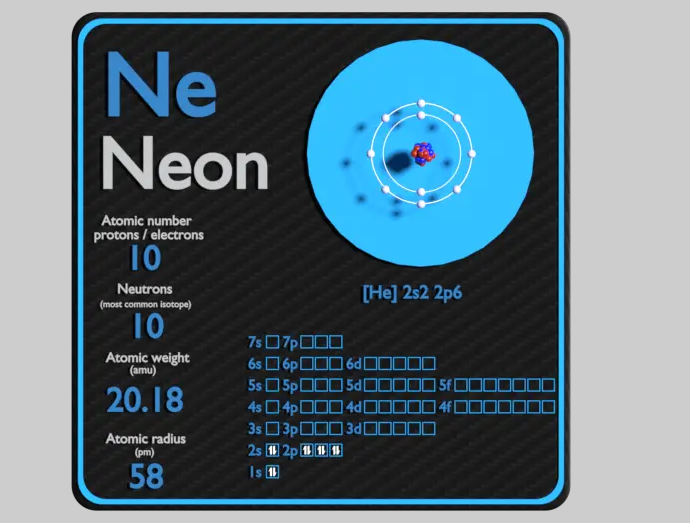
How many neutrons does neon have?
1. Introduction to Neon:
The first section serves as an introduction to neon, highlighting its properties, occurrence in nature, and various applications. We discuss its inert nature, which makes it suitable for lighting purposes, and its presence in the Earth's atmosphere.
2. The Basics of Atomic Structure:
To understand the number of neutrons in neon, it's essential to have a basic understanding of atomic structure. This section provides an overview of atoms, discussing their composition, including protons, neutrons, and electrons. We explain that protons and neutrons reside in the nucleus, while electrons orbit around it.
3. Neutrons in the Nucleus:
This section focuses on the role of neutrons in the nucleus of an atom. We explain that neutrons are subatomic particles with no charge, and their primary function is to stabilize the nucleus. We discuss how neutrons contribute to the overall mass of an atom.
4. Atomic Number and Mass Number:
To determine the number of neutrons in neon, we need to understand the concepts of atomic number and mass number. This section explains that the atomic number represents the number of protons in an atom, while the mass number represents the total number of protons and neutrons.
5. Neutrons in Neon:
Neon has an atomic number of 10, indicating that it contains 10 protons in its nucleus. This section reveals that neon's atomic mass is approximately 20.180 amu (atomic mass units). By subtracting the atomic number from the atomic mass, we can determine that neon has 10 neutrons.
6. Isotopes of Neon:
While neon typically has 10 neutrons, it's important to note that isotopes of neon exist with varying numbers of neutrons. This section briefly introduces the concept of isotopes and highlights a few examples of neon isotopes, such as neon-20, neon-21, and neon-22, which have 10, 11, and 12 neutrons, respectively.
7. Significance of Neutrons in Neon:
This section discusses the significance of neutrons in neon and their role in determining the stability and behavior of the element. We explore how the number of neutrons can affect the properties of isotopes and their applications in various fields.
8. Practical Applications of Neon:
While the focus of this article is on neutrons, we also briefly discuss practical applications of neon. This section highlights its use in lighting, lasers, cryogenics, and advertising signs, emphasizing its unique properties and versatility.
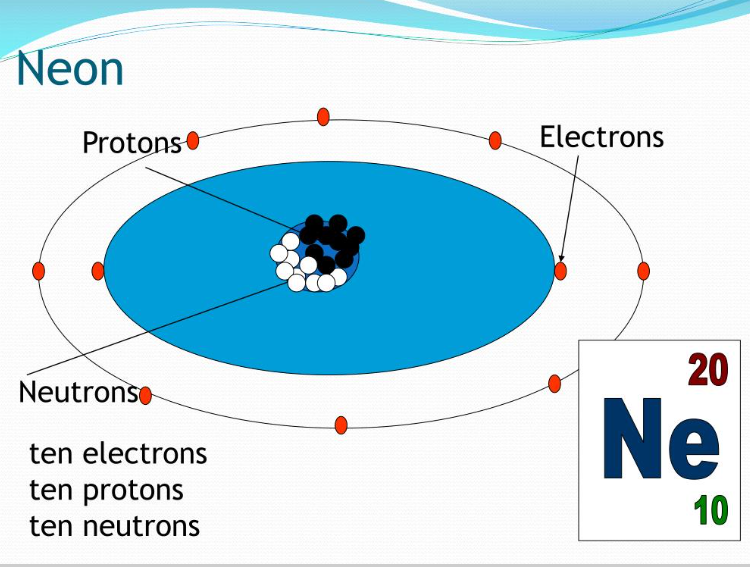
Neon
Neon, a noble gas element, contains 10 neutrons in its nucleus. By understanding the basic concepts of atomic structure, including the roles of protons, neutrons, and electrons, we can determine the number of neutrons in neon. Neutrons play a crucial role in stabilizing the nucleus and contribute to the overall mass of the element. As you marvel at the vibrant glow of neon lights or encounter neon in various applications, appreciate the intricate atomic structure that underlies its fascinating properties.