Which Symbol in a Chemical Equation Separates the Reactants from the Products?
Chemical equations are fundamental tools used in chemistry to represent chemical reactions. They provide a concise and symbolic way to express the transformation of reactants into products during a chemical process. Understanding the symbols used in chemical equations is essential for students, scientists, and anyone working with chemical reactions. In this article, we will explore the symbol that separates the reactants from the products in a chemical equation and its significance in conveying essential information about the reaction.
I. Chemical Equations: An Overview
Before delving into the specific symbol, let's briefly recap the structure of a chemical equation. A chemical equation consists of reactants on the left-hand side and products on the right-hand side, separated by an arrow.
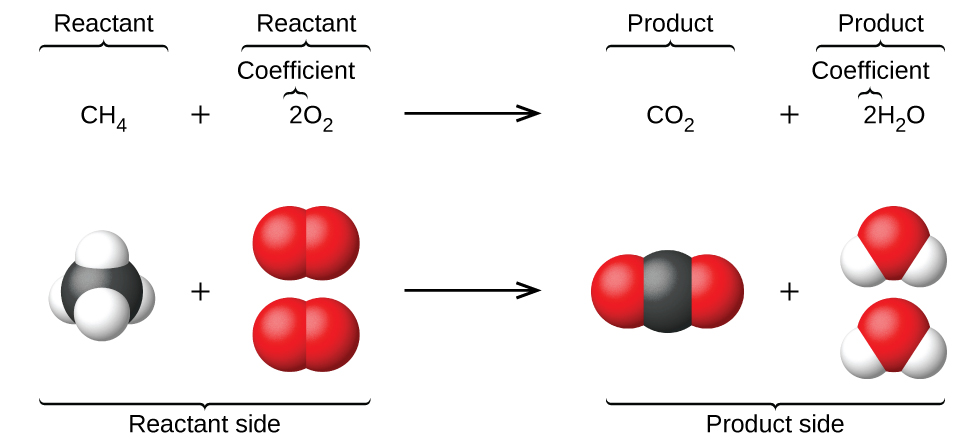
Chemical Equations
The arrow indicates the direction of the reaction, from reactants to products. These equations serve as a written representation of what happens in a chemical reaction, providing crucial information about the substances involved and the quantities of each.
II. The Separating Symbol: = → > +
In a chemical equation, the symbol that separates the reactants from the products is the arrow (→). This arrow is a key element of the equation, indicating the direction in which the reaction proceeds. It demonstrates that the reactants undergo a transformation and give rise to the products listed on the right side.
Additionally, there are a few other symbols that might be used in chemical equations, such as the equal sign (=), the greater than sign (>), and the plus sign (+).
1. Equal Sign (=): The equal sign is sometimes used in chemical equations to represent a reversible reaction. This means that the reaction can proceed in both directions, from reactants to products and from products to reactants.
2. Greater Than Sign (>): The greater than sign is occasionally used to signify irreversible reactions. In this context, the reaction proceeds only from reactants to products and not in the reverse direction.
3. Plus Sign (+): The plus sign in a chemical equation is used to separate two or more reactants or products that are present on either side of the equation. It indicates that these substances are combined or produced simultaneously in the reaction.
III. Significance of the Arrow and Other Symbols
The arrow and additional symbols in a chemical equation play a crucial role in conveying essential information about the reaction. Here's why they are significant:
1. Direction of the Reaction: The arrow (→) clearly shows the direction in which the chemical reaction proceeds, from the reactants on the left to the products on the right. This helps chemists understand the pathway of the reaction and predict the outcome.
The arrow
2. Reversibility: The use of the equal sign (=) indicates that the reaction is reversible, meaning it can proceed in both directions. This information is vital for understanding the equilibrium state of a reaction and the conditions under which it reaches equilibrium.
3. Irreversibility: On the other hand, the greater than sign (>) signifies that the reaction is irreversible and proceeds only in one direction. This understanding is essential in processes where the reaction is intentionally driven to completion.
4. Multiple Reactants and Products: The plus sign (+) is valuable when a chemical equation involves multiple reactants or products. It simplifies the representation of complex reactions, making it easier to comprehend and balance the equation.
IV. Conclusion
Chemical equations are indispensable tools for chemists to represent and understand chemical reactions. The arrow symbol (→) is fundamental in separating the reactants from the products and indicating the direction of the reaction.
Additionally, the use of other symbols, such as the equal sign (=), greater than sign (>), and plus sign (+), conveys further information about the reversibility and complexity of the reaction. By familiarizing ourselves with these symbols and their significance, we can gain a deeper understanding of chemical equations and the processes they represent.