Understanding the Role of Protons, Neutrons, and Electrons in the Atom
Atoms are the fundamental building blocks of matter, and understanding their composition is essential to comprehend the workings of the universe. At the core of every atom lies a nucleus, consisting of protons and neutrons, while electrons orbit around it. In this article, we will delve into the characteristics, roles, and interactions of protons, neutrons, and electrons, shedding light on their significance in atomic structure and behavior. By the end, you will have a comprehensive understanding of the role each of these particles plays in forming and defining the properties of matter.
1. Protons: The Positive Core
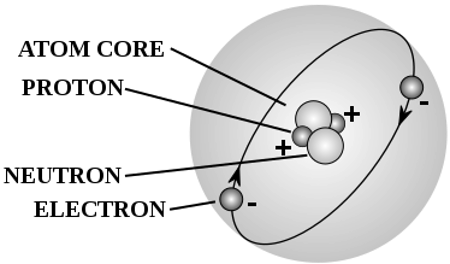
Protons: The Positive Core
Protons are positively charged particles found within the nucleus of an atom. They have a mass of approximately 1 atomic mass unit (AMU) and carry a unit positive charge. The number of protons in an atom determines its atomic number, uniquely identifying each element. In the periodic table, elements are arranged in ascending order based on their atomic number. Protons play a crucial role in defining an element's chemical properties, including its reactivity and bonding behavior. By altering the number of protons, different elements are formed, each with distinct characteristics.
2. Neutrons: Balancing the Nucleus
Neutrons: Balancing the Nucleus
Neutrons are uncharged particles present in the nucleus alongside protons. They have a mass of approximately 1 AMU, similar to protons. The number of neutrons can vary within a specific element, resulting in isotopes with different atomic masses. Neutrons serve as stabilizers within the nucleus, counterbalancing the electromagnetic repulsion between positively charged protons. This delicate balance between protons and neutrons determines the stability of an atom. Additionally, isotopes with varying numbers of neutrons may exhibit different nuclear properties, such as stability, radioactivity, and nuclear decay.
3. Electrons: Orbiting Clouds of Negativity
Electrons are negatively charged particles that orbit around the nucleus in specific energy levels or shells. These shells are further divided into subshells and atomic orbitals. Electrons possess negligible mass compared to protons and neutrons and are responsible for an atom's size and chemical reactivity. The outermost energy level, known as the valence shell, determines the atom's interaction with other atoms, leading to the formation of compounds and chemical bonding. The arrangement and distribution of electrons in an atom are governed by quantum mechanics, following specific rules such as the Pauli exclusion principle and Hund's rule.
4. Interactions and Bonding
The interplay between protons, neutrons, and electrons governs the behavior of atoms and their interactions with one another. Electrons are involved in chemical bonding, where they are shared, gained, or lost between atoms to achieve a stable configuration. This process leads to the formation of molecules and compounds. The number of valence electrons influences the type and strength of bonding an element can form, such as ionic, covalent, or metallic bonds. Protons and neutrons contribute to the stability and isotopic variations of elements, affecting their nuclear properties.
Protons, neutrons, and electrons are the fundamental particles that comprise atoms. Protons define an element's identity, neutrons contribute to stability, and electrons determine an atom's size and reactivity. Understanding the roles and interactions of these particles provides insight into atomic structure, chemical properties, and the formation of compounds. By manipulating the number of protons, neutrons, and electrons, scientists can create isotopes and study various aspects of atomic behavior. This knowledge serves as the foundation for fields like chemistry, physics, and materials science, enabling us to unlock the secrets of the microscopic world.