Understanding Phenol (C6H5OH) and Its Reaction with Bromine (Br2)
Phenol, with its distinct chemical structure and reactivity, plays a significant role in organic chemistry. When it encounters bromine (Br2), a halogen, interesting chemical reactions occur. In this article, we will explore the properties of phenol and delve into the reaction it undergoes with bromine, shedding light on the essential aspects of this chemical interaction.
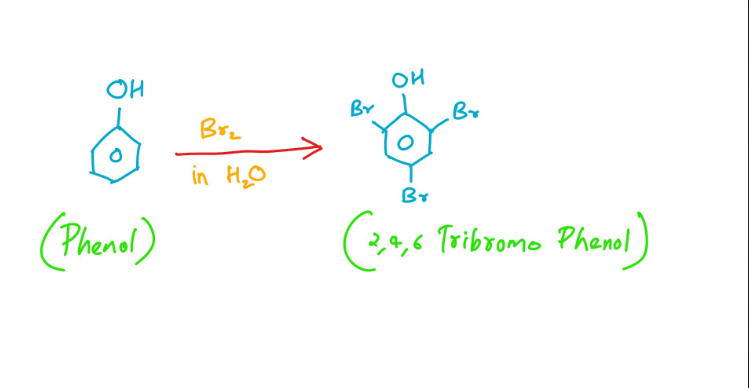
Phenol Br2 in water
1. Introduction to Phenol (C6H5OH)
a. Chemical Structure: Phenol is a chemical compound consisting of a phenyl group (C6H5) bonded to a hydroxyl group (OH). This unique structure sets it apart from other aromatic compounds.
b. Occurrence: Phenol can be found naturally in some plants, but it is primarily produced synthetically for various industrial applications.
2. The Chemistry of Phenol
a. Acidic Nature: Phenol is mildly acidic due to the presence of the hydroxyl group. It can donate a proton (H+) to form phenoxide ions.
b. Aromatic Ring: The phenyl ring in phenol is aromatic, meaning it has a stable, resonance-enhanced electron cloud.
3. Bromine (Br2) as a Halogen
a. Properties: Bromine is a halogen known for its distinctive reddish-brown liquid state at room temperature. It is highly reactive and readily forms compounds with other elements.
b. Use in Organic Chemistry: Bromine is often used as a reagent in organic synthesis to introduce bromine atoms into organic molecules.
4. Reaction of Phenol with Bromine (Br2)
a. Bromination Reaction: When phenol reacts with bromine (Br2) in the presence of a Lewis acid catalyst, such as iron(III) bromide (FeBr3), a bromination reaction occurs.
b. Electrophilic Aromatic Substitution: The bromination of phenol is an example of electrophilic aromatic substitution. The electrophilic bromine atom (Br+) attacks the electron-rich aromatic ring of phenol.
5. Mechanism of the Bromination Reaction
a. Formation of Electrophile: The Lewis acid catalyst (FeBr3) facilitates the formation of an electrophilic bromine species (Br+).
b. Attack on the Phenyl Ring: The electrophilic bromine attacks the phenyl ring, leading to the substitution of one hydrogen atom with a bromine atom.
c. Generation of Hydrogen Bromide: As a result of the substitution, hydrogen bromide (HBr) is produced as a byproduct.
6. Significance of the Bromination of Phenol
a. Introduction of Bromine: The reaction results in the introduction of a bromine atom into the phenol molecule.
b. Altered Reactivity: The presence of the bromine atom can alter the reactivity and properties of the phenol compound.

Phenol Br2
Phenol, with its unique chemical structure and reactivity, undergoes a fascinating bromination reaction with bromine (Br2) in the presence of a Lewis acid catalyst. This reaction is a prime example of electrophilic aromatic substitution, leading to the introduction of a bromine atom into the phenol molecule. Understanding such chemical interactions is crucial in the realm of organic chemistry, as it allows scientists to manipulate and synthesize various compounds for a wide range of applications, from pharmaceuticals to materials science. The bromination of phenol is just one of many intriguing reactions that contribute to our understanding of the chemical world.