Understanding the Role and Count of Valence Electrons in Nickel
In the realm of chemistry and physics, the concept of valence electrons holds paramount importance. These electrons, found in the outermost shell of an atom, play a crucial role in determining an element's chemical properties and reactivity. In this article, we delve into the world of nickel, a transition metal, and explore the significance of its valence electrons.
I. Understanding Valence Electrons
Valence electrons are the electrons located in the outermost energy level of an atom. They are instrumental in the formation of chemical bonds and interactions with other atoms. The number of valence electrons largely dictates an element's placement in the periodic table and its chemical behavior. For transition metals like nickel, the understanding of valence electrons becomes even more intriguing due to their unique electronic configurations.
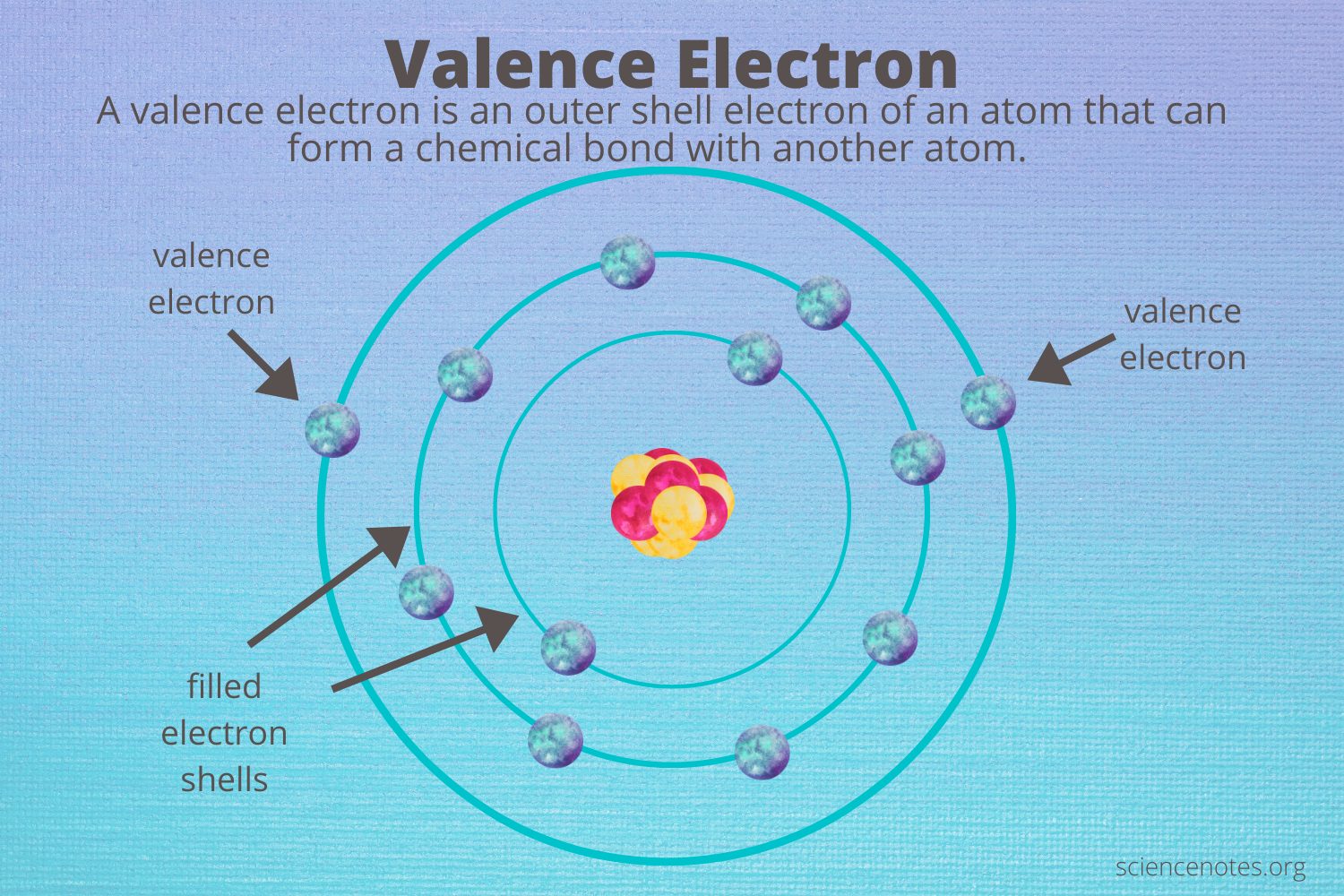
Valence electrons
II. Nickel's Atomic Structure
Nickel, with the atomic number 28 and symbol Ni, boasts a complex atomic structure that contributes to its diverse range of applications. Its electron configuration is [Ar] 3d8 4s2, revealing that nickel has 8 electrons in its d orbital and 2 in the outermost s orbital. This electron arrangement places nickel in the transition metal category, positioning it between highly reactive alkali metals and relatively inert noble gases in the periodic table.
III. The Role of Valence Electrons in Nickel's Chemistry
Nickel's valence electrons, specifically those in the 4s and 3d orbitals, define its chemical behavior. With 2 valence electrons in the 4s orbital, nickel can readily lose these electrons to attain a stable electron configuration similar to that of the preceding noble gas, argon. This characteristic enables nickel to exhibit varying oxidation states, commonly +2 and +3, allowing it to form compounds with different elements.
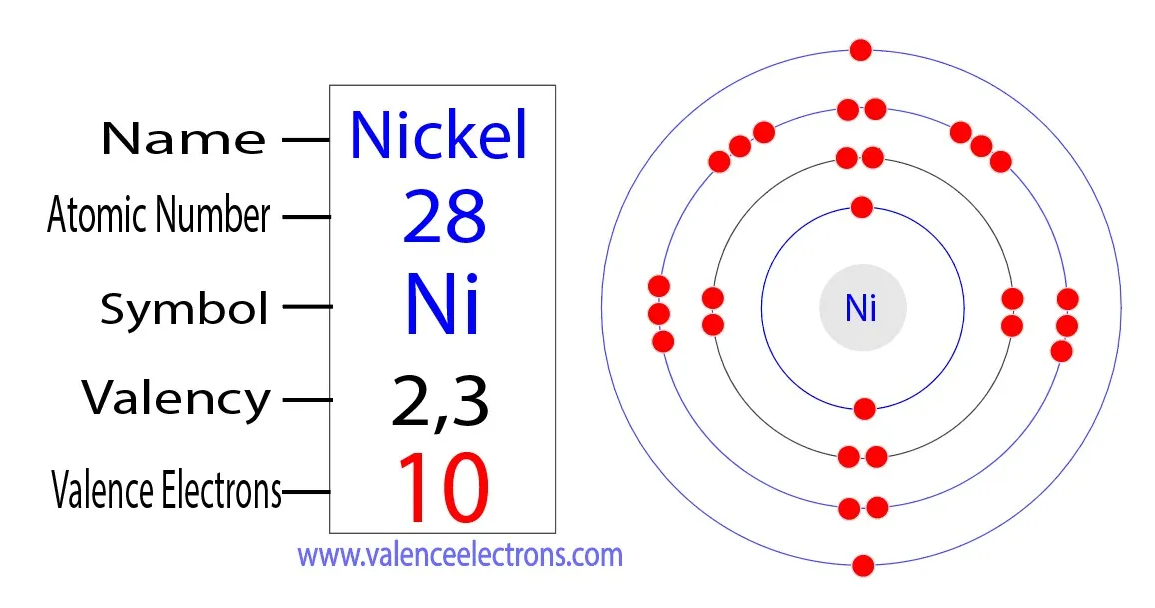
Valence Electrons in Nickel
IV. Importance in Catalysis
One of the prominent applications of nickel lies in catalysis. The unique configuration of its valence electrons grants nickel the ability to participate in various catalytic reactions. Nickel catalysts are extensively used in industrial processes like hydrogenation, where hydrogen molecules adsorb onto the nickel surface, undergo reactions, and ultimately lead to the desired product. This ability to adsorb and activate molecules is directly tied to nickel's valence electron configuration.
V. Valence Electrons and Magnetic Properties
Nickel is also recognized for its magnetic properties, making it a vital component in various magnetic alloys and materials. Its magnetic behavior is attributed to the arrangement of its valence electrons in the 3d orbitals. The presence of unpaired electrons in these orbitals allows nickel atoms to align their magnetic moments, contributing to the overall magnetism of the material.
VI. Valence Electrons' Influence on Nickel's Reactivity
The reactivity of nickel, particularly its susceptibility to corrosion, is intricately linked to its valence electrons. Nickel's ability to readily lose electrons and form positive oxidation states makes it susceptible to oxidation, leading to the formation of oxides on its surface. These oxides can impact the material's mechanical and chemical properties, a factor of utmost consideration in applications ranging from metallurgy to electronics.
VII. Conclusion
In the realm of chemistry, the valence electrons of nickel hold the key to its diverse properties and applications. From catalysis to magnetism, these electrons dictate how nickel interacts with its surroundings. Its electron configuration, [Ar] 3d8 4s2, bestows upon it a unique set of characteristics that scientists and engineers leverage for a wide array of technological advancements.
As we continue to uncover the intricate relationship between electron configurations and material properties, nickel stands as a shining example of the profound impact valence electrons can have in the world of elements.