Elements with Reactivity Similar to Chlorine (Cl): Exploring Chemical Analogues
Chemical reactivity, a fundamental property of elements, determines how they interact with other substances. Chlorine (Cl), a highly reactive element, exhibits unique characteristics in chemical reactions. In this article, we will explore the pair of elements that share similar reactivity to chlorine. By examining their properties, electronic configurations, and reactivity patterns, we aim to uncover the chemical analogues that closely resemble chlorine in terms of reactivity.
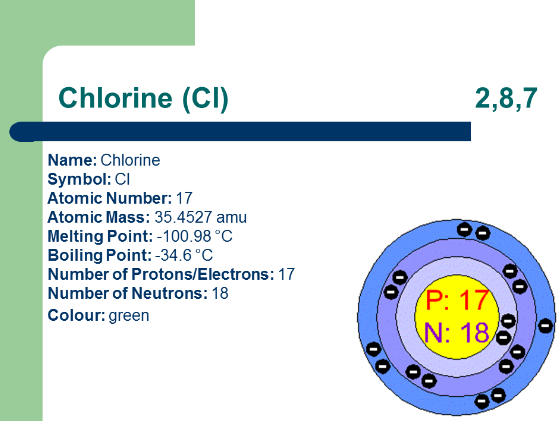
Information about Chlorine
I. Understanding Chlorine's Reactivity:
1.1 Overview of Chlorine (Cl): Introducing the properties and characteristics of chlorine. Highlighting its role as a halogen and its position in the periodic table.
1.2 Importance of Reactivity: Exploring the significance of reactivity in chemical interactions. Understanding how reactivity influences chemical bonding and reactions.
II. Identifying Elements with Similar Reactivity to Chlorine:
2.1 Group 17 Elements: The Halogens: Introducing the halogens and their position in the periodic table. Discussing the similarities in reactivity within Group 17.
2.2 Comparing Reactivity Patterns: Analyzing the reactivity trends of halogens, including chlorine. Examining the factors that influence reactivity within the group.
III. Pairing Chlorine with its Reactive Analogues:
3.1 Fluorine (F): Exploring the reactivity similarities between fluorine and chlorine. Highlighting the shared properties and characteristics.
3.2 Bromine (Br): Examining the reactivity parallels between bromine and chlorine. Contrasting the properties and differences between the two elements.
IV. Electronic Configurations and Reactivity:
4.1 Electronic Configuration of Chlorine: Understanding chlorine's electron arrangement in its atomic structure. Linking electronic configuration to reactivity patterns.
4.2 Electronic Configurations of Fluorine and Bromine: Comparing the electron configurations of fluorine and bromine with chlorine. Discussing how electronic structure contributes to reactivity similarities.
V. Applications and Uses of Chlorine and Its Analogues:
5.1 Chlorine's Applications: Examining the diverse industrial and household uses of chlorine. Highlighting its importance in water treatment and chemical synthesis.
5.2 Applications of Fluorine and Bromine: Exploring the practical applications of fluorine and bromine. Discussing their roles in various industries and sectors.
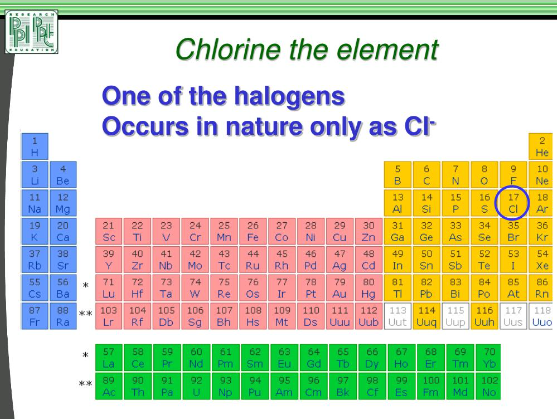
Chlorine the element
In conclusion, when searching for elements with reactivity similar to chlorine (Cl), we find that fluorine (F) and bromine (Br) closely resemble chlorine in their chemical behavior. These halogens share common properties, reactivity patterns, and electron configurations that contribute to their analogous behavior. Understanding the chemical analogues of chlorine enhances our comprehension of element reactivity, facilitating scientific research, industrial applications, and technological advancements.