The Cohesive Property of Water: Understanding its Responsible Factors
Water is a remarkable substance that exhibits several unique properties, including its ability to stick to itself, known as cohesion. In this article, we will explore the factors responsible for the cohesive property of water and delve into the science behind it. Understanding these factors will provide insights into the fascinating behavior of water molecules.
Which of the following is responsible for the cohesive property of water?
1. Definition of Cohesion:
Cohesion refers to the attraction between molecules of the same substance. In the case of water, it is the force that holds water molecules together, creating surface tension and allowing water to form droplets.
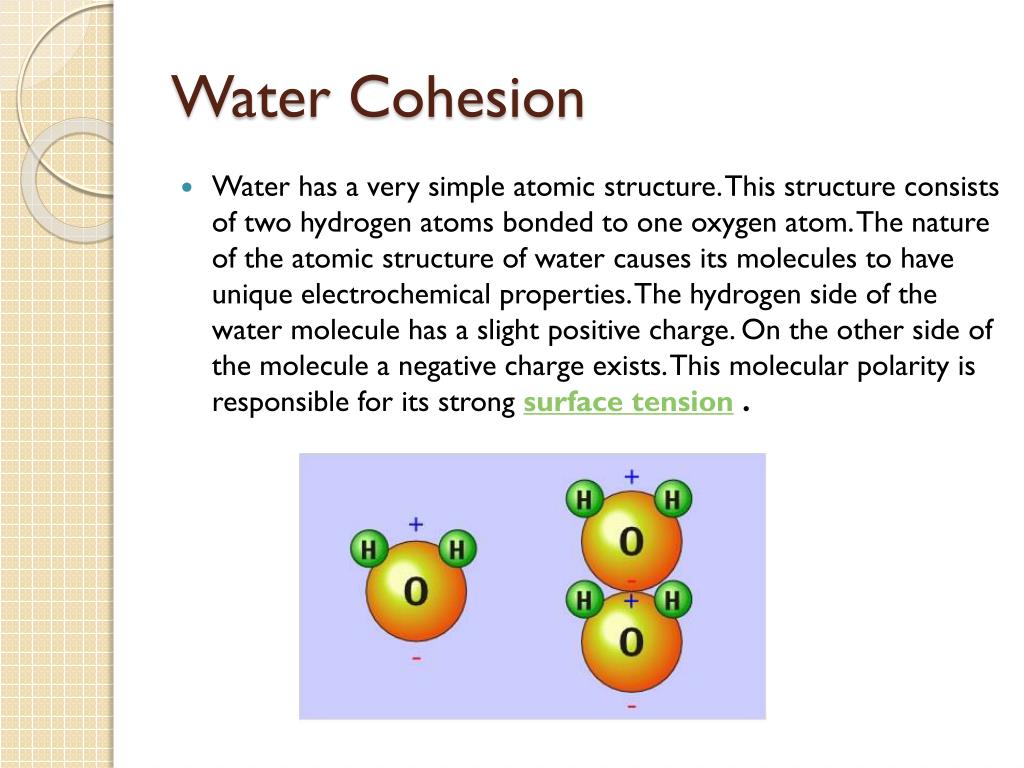
2. Hydrogen Bonding:
The primary factor responsible for the cohesive property of water is hydrogen bonding. Water molecules consist of two hydrogen atoms bonded to one oxygen atom. The oxygen atom has a slightly negative charge, while the hydrogen atoms have a slightly positive charge. This polarity enables water molecules to form hydrogen bonds with each other, creating a cohesive force.
3. Polarity of Water:
The polarity of water arises from the unequal sharing of electrons between oxygen and hydrogen atoms.
The oxygen atom is more electronegative, meaning it attracts electrons more strongly.
As a result, the oxygen end of the molecule becomes slightly negative, while the hydrogen ends become slightly positive. This polarity allows water molecules to align and form hydrogen bonds, contributing to cohesion.
4. Surface Tension:
The cohesive property of water gives rise to surface tension, which is the force that allows water to resist external forces. Water molecules at the surface experience a stronger attraction to neighboring molecules beneath them, causing them to be pulled inward. This creates a "skin" on the surface of the water and enables small objects, such as insects, to float on the water's surface.
5. Capillary Action:
Another phenomenon resulting from water's cohesive property is capillary action.
Capillary action is the ability of water to move against gravity in narrow spaces, such as thin tubes or porous materials.
The cohesive forces between water molecules allow them to "tow" each other upward, defying gravity. This property is crucial for plants to transport water from the roots to the leaves.
6. Temperature and Cohesion:
Temperature plays a role in the cohesive property of water.
As temperature decreases, water molecules slow down, and hydrogen bonds become stronger, leading to increased cohesion.
This is why water forms ice, which has a highly ordered structure due to extensive hydrogen bonding. In contrast, higher temperatures can weaken hydrogen bonds, reducing cohesion.
7. Importance of Cohesion:
The cohesive property of water has significant implications in various aspects of life. It enables water to flow in plants, aids in the movement of blood in animals, and facilitates the transport of nutrients and waste in living organisms. Cohesion also contributes to the formation of raindrops, the shaping of water bodies, and the functioning of many natural processes.

Water Cohesive
The cohesive property of water is a fascinating characteristic that arises from hydrogen bonding and the polarity of water molecules. This property allows water to stick to itself, creating surface tension, capillary action, and other important phenomena. Understanding the factors responsible for water's cohesion provides insights into the behavior of this vital substance and its crucial role in the natural world.