What Temperature Does Alcohol Evaporate? Exploring the Science Behind Evaporation
Alcohol is a common household substance that is widely used for various purposes, including cooking, cleaning, and recreational activities. One intriguing aspect of alcohol is its ability to evaporate at certain temperatures. In this article, we will delve into the science behind alcohol evaporation and explore the specific temperature at which it occurs.

Alcohol Evaporation
Understanding Evaporation
Evaporation is a natural process by which a liquid transforms into a gaseous state. It involves the escape of molecules from the liquid's surface into the surrounding air. Several factors influence the rate of evaporation, including temperature, surface area, humidity, and the nature of the liquid itself.
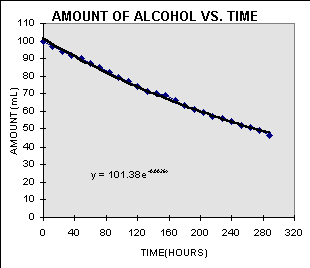
The Basics of Alcohol Evaporation
Alcohol, or ethanol, has a lower boiling point compared to water, making it evaporate at a lower temperature. While water boils at 100 degrees Celsius (212 degrees Fahrenheit), ethanol has a significantly lower boiling point of approximately 78.4 degrees Celsius (173.12 degrees Fahrenheit). Consequently, alcohol evaporates faster than water when exposed to the same conditions.
Factors Affecting Alcohol Evaporation
1. Temperature:
The rate of alcohol evaporation is primarily influenced by the surrounding temperature.
As the temperature increases, more alcohol molecules gain sufficient energy to break free from the liquid's surface, resulting in faster evaporation.
Conversely, colder temperatures slow down the process of alcohol evaporation.
2. Alcohol Concentration:
The concentration of alcohol in a solution also affects its evaporation rate.
Higher alcohol concentrations tend to evaporate more quickly than solutions with lower alcohol content.
This is because the presence of other substances, such as water or solvents, can hinder the evaporation process.
3. Surface Area:
The surface area of the liquid exposed to the air plays a crucial role in evaporation.
A larger surface area allows for more alcohol molecules to escape into the surrounding air, leading to faster evaporation.
For example, pouring alcohol into a shallow dish will result in faster evaporation compared to leaving it in a sealed bottle.
Alcohol Evaporation
Applications of Alcohol Evaporation
1. Cooking:
Alcohol is commonly used in cooking to enhance the flavor of various dishes.
When added to a recipe, alcohol evaporates during the cooking process, leaving behind the desired flavors without the alcoholic content.
Understanding the temperature at which alcohol evaporates can help chefs determine the optimal time to add alcohol to their dishes.
2. Cleaning:
Alcohol is an effective cleaning agent due to its ability to evaporate quickly.
It is often used to disinfect surfaces, remove stains, and sanitize objects.
Knowing the evaporation temperature of alcohol can assist in selecting the appropriate concentration and application method for cleaning purposes.
3. Distillation:
Distillation is a process that utilizes the different boiling points of substances to separate them.
Alcohol distillation involves heating a mixture to vaporize the alcohol, which is then condensed and collected.
The knowledge of alcohol's evaporation temperature is crucial for achieving successful distillation results.
Safety Considerations
While alcohol evaporation has numerous practical applications, it is important to consider safety precautions.
Alcohol vapors can be flammable, so it is essential to avoid open flames or sparks in areas where alcohol is being evaporated.
Adequate ventilation should also be provided to prevent the buildup of potentially harmful vapors.
Understanding the temperature at which alcohol evaporates is essential for various applications, ranging from cooking to cleaning and distillation. With a lower boiling point than water, alcohol evaporates at a faster rate when exposed to the appropriate conditions. By considering factors such as temperature, alcohol concentration, and surface area, individuals can make informed decisions regarding the evaporation of alcohol. However, it is crucial to prioritize safety and take necessary precautions when handling alcohol and its vapors.